Difference between homogeneous and heterogeneous mixture Explanation Homogeneous mixtures The homogeneous mixture has the same uniform appearance and overall compositionIt has only one phase Many homogeneous mixtures are generally referred to as solutions Examples Corn oil, White vinegar, A sugar solution and Air with no clouds Heterogeneous mixtures A heterogeneous mixtureHomogeneous mixtures have the same composition throughout the system, and heterogeneous mixturesDifference between homogeneous and heterogeneous mixture 1) A homogeneous mixture has a uniform composition throughout its mass whereas a heterogeneous mixture has a non uniform composition 2) A homogeneous mixture does not contain physically distinct parts whereas a heterogeneous mixture contains physically distinct parts

Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet By Elly Thorsen
Difference between heterogeneous and homogeneous mixture
Difference between heterogeneous and homogeneous mixture-Difference between Homogeneous and Heterogeneous Mixture Homogeneous mixture Heterogeneous mixture It can't be separated out physically It can be separated out physically 'homo' means the same 'hetero' means different Example a mixture of alcohol and water Example a mixture of sodium chloride and sandHomogeneous mixtures have uniform composition Heterogeneous mixtures have nonuniform composition




Chemistry For Kids Chemical Mixtures
The main conflict between them is that all the components are dissolved and cannot be taken out in a homogeneous mixture while in Heterogeneous mixture components are not dissolved equally and we can separate themTo summarise, Earlier, homogenous was used as a scientific term, mainly in biology, but now it is almost obsolete, being replaced by homologous Whereas, homogeneous is a common word, very much in use and means having similar or comparable characteristics Rate this article (9 / 56 votes) Email PrintHomogeneous Mixtures Homogenous mixtures have a uniform composition throughout the mixture Heterogeneous Mixtures Heterogenous mixtures have a mixed composition which may vary from point to point
A heterogeneous mixture is a mixture that is in at least two different states of matter (two phases), its components are mixed in a nonuniform way and it is possible to differentiate them with the naked eye While in a homogeneous mixture the components are distributed in the same way, in any region of the mixture, in a heterogeneous mixtureWhile heterogeneous mixtures are uneven, with a composition that varies from one point to another In homogeneous mixtures, there seems to be only one component (solute and solvent), but in heterogeneous, we easily visualize more than two components5 σειρέςThe components of homogeneous mixtures are not physically distinct A heterogeneous mixture
How are they different?A homogeneous mixture has a uniform composition and appearance Individual substances that constitute a homogeneous mixture cannot be visually differentiated On the other hand, a heterogeneous mixture comprises two or more substances that can be distinctly observed, and even separated relatively easilyThe difference between heterogeneous and homogeneous mixtures is the degree to which materials are mixed together and the uniformity of their composition A Homogeneous mixture is a mixture in which components that make up mixture are uniformly distributed throughout the mixture The composition of the mixture is same throughout




What Is Difference Between Heterogeneous And Homogeneous Brainly In




Teks Describe Heterogeneous And Homogeneous Mixtures 6d Explain The Similarities And Differences Between Heterogeneous And Homogenous Mixtures 6e Ppt Download
Difference between Homogeneous and Heterogeneous HOMOGENEOUS Difference between Homogeneous and Heterogeneous Homogeneous refers to the solution that results from a completely uniform mixture of two or more elements In a homogeneous mixture, all the elements come together to the point where it becomes difficult to distinguish one from theA homogenous mixture is defined as the mixture in which the composition formed is uniform On the other hand, a heterogeneous mixture is defined as the mixture in which the composition formed is not uniform 2 The elements and substances used in homogenous mixturesThe Difference Between Heterogeneous and Homogeneous Mixtures Homogeneous Mixture Examples You can't pick out components of a homogeneous mixture or use simple mechanical means to Heterogeneous Mixture Examples Heterogeneous mixtures are more common than homogeneous mixtures Usually,




Types Of Mixtures Difference Between Homogeneous Mixture Heterogeneous Mixture Youtube




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
A mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by physical changes, such as evaporation A mixture with a composition that varies from point to point is called a heterogeneous mixture A homogeneous mixture, also called a solution exhibits a uniform composition and appearsDifference Between Pure Substance and Homogeneous Mixture Pure Substance vs Homogeneous Mixture Matter is composed of different substances, like atoms and other molecules, that have volume and mass All physical objects are made up of chemical substances which are unchanging in chemical composition and characteristics A chemical substance is alsoA mixture is formed by combining two or more materials A homogeneous mixture appears uniform, regardless of where you sample it A heterogeneous mixture contains particles of different shapes or sizes and the composition of one sample may differ from that of another sample




Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture Venn Diagram Examples Venn Diagram Template



Homogeneous Heterogeneous Mixture Definition Examples Selftution
Heterogeneous mixture In heterogeneous mixtures, the compounds that form them repel each other due to the nature of their bonds, as is the case of water and oil Below are the differences between homogeneous and heterogeneous mixtures Visibility In the heterogeneous mixtures, the components that make up the mixture can be clearly seenDistinguishing between mixture types Making a distinction between homogeneous and heterogeneous mixtures is a matter of the scale of sampling On a coarse enough scale, any mixture can be said to be homogeneous, if the entire article is allowed to count as a sample of itDifference between Homogeneous and Heterogeneous Key Difference Homogenous refers to a solution that is a completely uniform mixture of two or more objects Heterogeneous refers to solutions that are not completely uniform and in most cases is clearly visible when viewing the mixture The terms 'homogeneous' and 'heterogeneous' are




Chemistry For Kids Chemical Mixtures



Is There Any Difference Between Homogeneous Mixture And Solution Here On Quora Previous Answers Are Vague About This While All My Textbooks And Google Sites Say They Are Exactly Same Quora
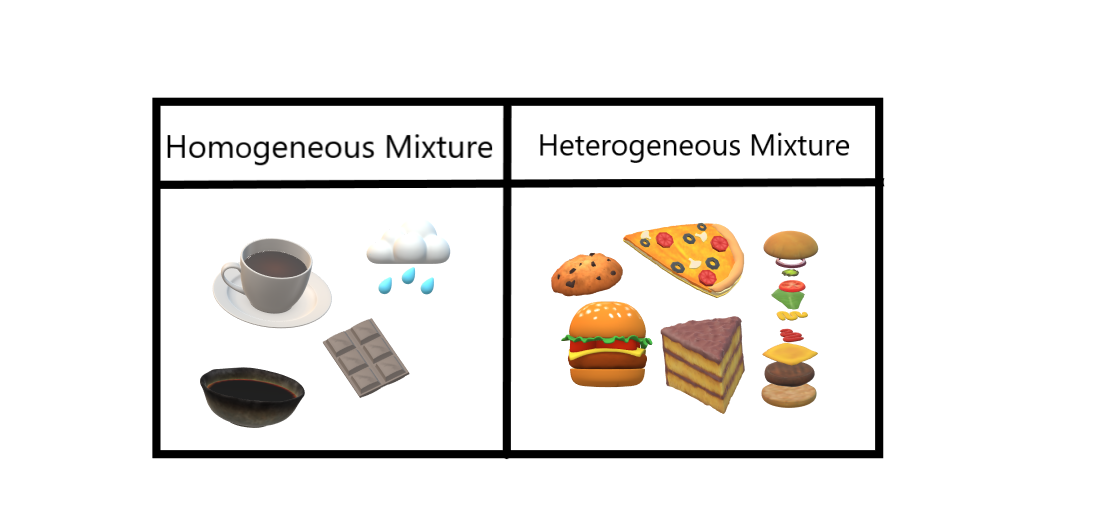
Definition A homogenous mixture is the type of mixture in which the composition of the solute is uniform throughout the mixture The heterogeneous mixture is the type of mixture in which the composition of the solute is not uniform throughout the mixture Particle sizeWhat is the difference between homogeneous and heterogeneous mixtures Get the answers you need, now!A homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases Solutions have particles which are the size of atoms or molecules too small to be seen




Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet Teaching Chemistry Middle School Science Experiments Middle School Science Resources




Give Three Differences Between Homogeneous Mixture And Heterogeneous Mixture Brainly In
The key difference between homogeneous and heterogeneous equilibrium is that in homogeneous equilibrium, the reactants and products are in the same phase of matter whereas, in heterogeneous equilibrium, the reactants and products are in different phases Equilibrium is a state in which the concentrations of reactants and products remain constantIn this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference betweenAnswer Difference between homogeneous and heterogeneous mixture are 1) A homogeneous mixture has a uniform composition throughout its mass whereas a heterogeneous mixture has a non uniform composition 2) A homogeneous mixture does not contain physically distinct parts whereas a heterogeneous mixture contains physically distinct parts
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Difference Between Homogeneous And Heterogeneous Mixture Brainly In
Homogeneous mixtures are uniform, that is, their composition is the same wherever you look at it;Homogeneous mixtureHeterogeneous mixture1) These are called as solutionsThese are called asHomogenous Equilibria A homogeneous equilibrium is one in which all of the reactants and products are present in a single solution (by definition, a homogeneous mixture ) Reactions between solutes in liquid solutions belong to one type of homogeneous equilibria The chemical species involved can be molecules, ions, or a mixture of both




Homogeneous Mixture Definition Examples Tutors Com




Zline3bn7w 3ym
Homogeneous mixtures have only one phase of matter, be it solid, liquid or gas Heterogeneous mixtures have more than one phases of matter It should be reminded here, that non mixing liquids are not considered 'one phase' For solutions in a trThe difference between heterogeneous and homogeneous mixtures is the degree to which materials are mixed together and the uniformity of their composition A Homogeneous mixture is a mixture in which components that make up mixture are uniformly distributed throughout the mixture The composition of the mixture is same throughoutA heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture The composition varies from one region to another with at least two phases that remain separated from each other, with clearly identifiable properties Examples Include Mud and water;




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science




Heterogeneous Mixtures Homogeneous Mixtures By Ms Cunningham
This video is in simple language about Difference between homogenous and heterogeneous mixturesClass 9Chapter 2Is Matter Around Us PureAir with cloud ;The prefixes hetero indicate difference A homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases




Heterogeneous And Homogeneous Mixtures What S The Difference Homogeneous Mixture Heterogeneous Mixture Chemistry




Difference Between Homogeneous And Heterogeneous Mixture Snapsolve
Homogeneous Mixture Vs Heterogeneous Mixture The comparison between heterogeneous and homogenous mixtures presented here, will clear all doubts regarding the differences between these two types These are two of most fundamental concepts in chemistry6 σειρέςThe homogeneous mixture is only in the one phase of matter, whereas heterogeneous mixture isDifferentiate between Homogeneous and Heterogeneous mixture with examples by Jaishree Gorane Leave a Comment List the points of difference between Homogeneous and Heterogeneous mixtures
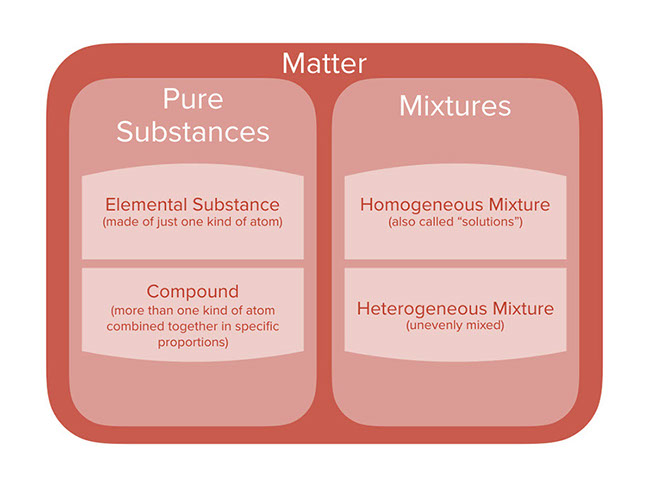



Lesson Categories Of Chemicals And Mixtures




Hetrogenous And Homogenous Mixture Ppt
A mixture of alcohol and water is homogeneous while that of oil and water is heterogeneous Explain asked in Class IX Science by ashu Premium ( 930 points)A mixture in which different constituents are mixed uniformly, is heterogeneous mixture 2 The component of a homogeneous mixture cannot seen by naked eye The component of a heterogeneous mixture cannot be seen by naked eye 3 Example of Homogeneous mixture sugar solution Example of Heterogeneous mixture Sand and Iron fillingA mixture is a combination of two or more substances that are not chemically united Mixtures can be homogeneous and heterogeneous, two terms that are very common in chemistry If you're trying to learn more about science, you'll need to be familiar with the definition of homogeneous and heterogeneous mixtures and the differences between them




Homogeneous Mixture Definition Examples Tutors Com




Difference Between Homogeneous And Non Homogeneous Mixtures Brainly In
6 σειρέςMixtures can be classified as either heterogeneous or homogeneous Homogeneous mixtures have aA homogeneous mixture has only one phase throughout the solution while a heterogeneous mixture has more than one distinct phases in the mixture A phase, at least in terms of mixture, is a region in the mixture where the composition is constant wherever in that region In a homogeneous mixture (may also be called a solution), the whole of the mixture hasCompound they are basically the pure substances composed of two or more different types of elements which combine in fixed proportions by mass Eg H2O where the mass of hydrogen is 2g and oxygen is 16g So, 216 = 18(on cancelling) Mixture it'




Teks Describe Heterogeneous And Homogeneous Mixtures 6d Explain The Similarities And Differences Between Heterogeneous And Homogenous Mixtures 6e Ppt Download




Mixture Wikiwand
The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties Homogeneous and heterogeneous are two different words that we can distinguishMixture of oil and water;Heterogeneous mixture (I) Homogeneous mixtures have uniform composition throughout the mixture (II) The whole mixture is in same phase (III) Components are not visible to the naked eye (IV) Components cannot be separated easily Eg Sugar Water → Sugar solution (I) Heterogeneous mixture have composition which may vary from point to point



Difference Between Homogenous And Heterogeneous Mixture Javatpoint




1 Classify The Following Liquid Mixtures As Chegg Com
A mixture is basically a combination in which two or more substances are combined A solution is mainly of two types that are homogeneous mixtures and heterogeneous mixtures A solution is a homogeneous mixture of solute and a solvent A solute is a substance that gets dissolved in a solvent whereas the solvent is the substance that dissolves aThe difference between homogeneous mixtures and heterogeneous mixtures is a matter of scale the heterogeneous mixture can be seen on beaches where sand included many particles like coral, shells and organic matter, etc they all can be separated easily hence known as a heterogeneous mixture but when we take a large amount of sand, it's



Mixture




Properties Of Homogeneous Mixture The Difference Between Heterogeneous And Homogeneous Mixtures



Yhs Apsva Us Wp Content Uploads Legacy Assets Yhs Dc39e Lecture2hw Pdf




Classification Of Matter Chemistrygod



Homogeneous And Heterogeneous Mixtures Geeksforgeeks



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Heterogeneous And Homogeneous Mixtures Odd One Out Worksheet By Elly Thorsen
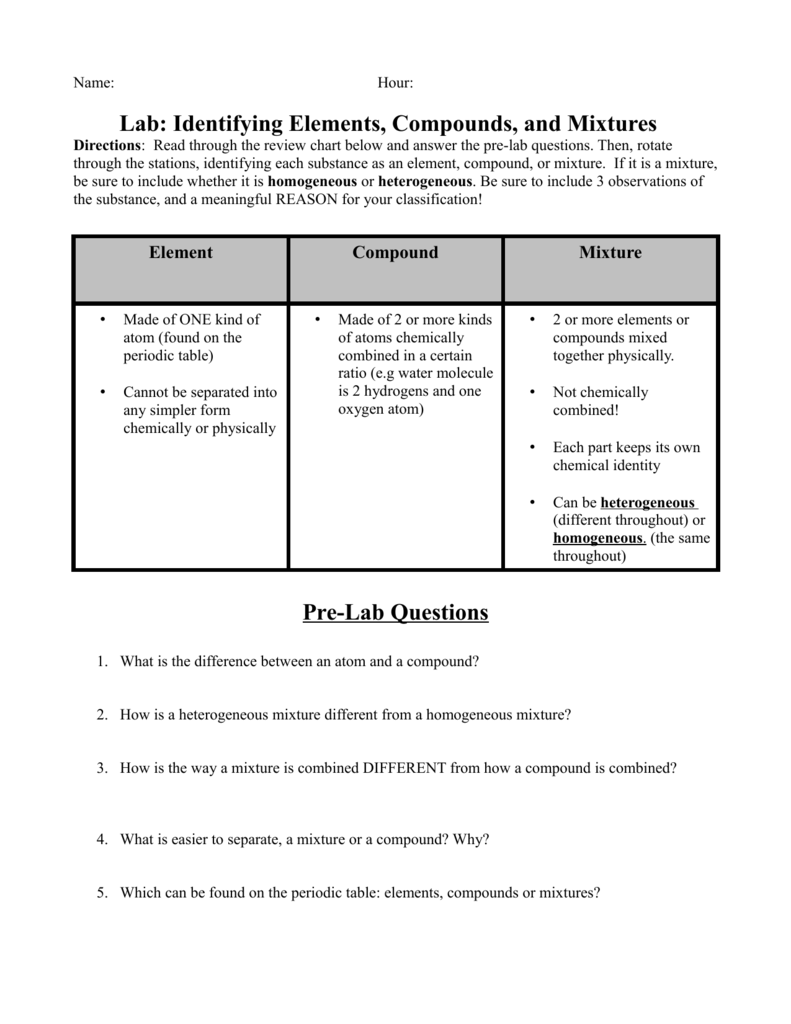



Lab Identifying Elements Compounds And Mixtures Pre




What Is A Heterogeneous Mixture Definition And Examples




Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous Vs Heterogeneous Youtube
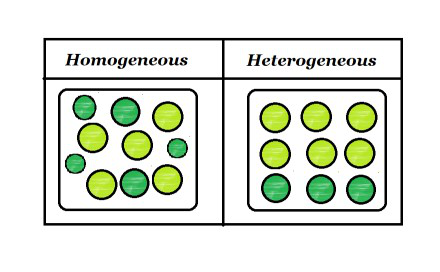



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Q2 Differentiate Between Homog Lido




Homogenous Vs Homogeneous What S The Difference Writing Explained




Compound Vs Mixture Difference And Comparison Diffen




Hetrogenous And Homogenous Mixture Ppt




10 Examples Of Mixtures




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms




Interesting Chemistry Difference Between Homogeneous And Heterogeneous Mixture Chemical Chemistrynotes Chemistry Facebook




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous




Do Now Today We Will Be Discussing The Differences Between Heterogeneous And Homogeneous Mixtures Copy The Following The Words Heterogeneous And Homogeneous Ppt Download
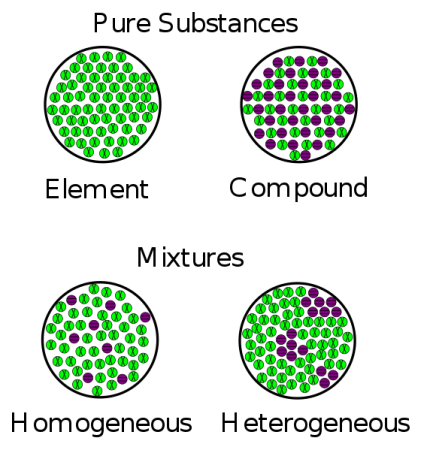



Heterogeneous Mixture Definition Science Trends




A State The Main Points Of Difference Between Homogenous And Hetergeneous Mixtures Youtube




Pdf Do Now Today We Will Be Discussing The Differences Between Heterogeneous And Homogeneous Mixtures Copy The Following The Words Course Hero




Chrominfo Difference Between Suspension And Solution
-and-Milk-(left).jpg?revision=1)



1 3 Classification Of Matter Chemistry Libretexts




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures



3




Heterogeneous And Homogeneous Mixture Differences Videos Examples




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry




Heterogeneous And Homogeneous Mixtures Activity Worksheets Tpt
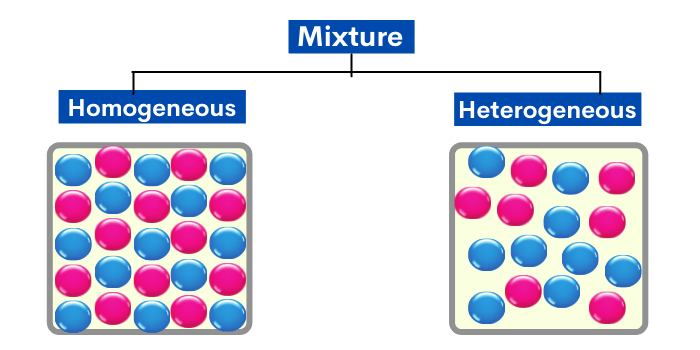



What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples



What Is Homogeneous And Heterogeneous System Quora




Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet By Elly Thorsen
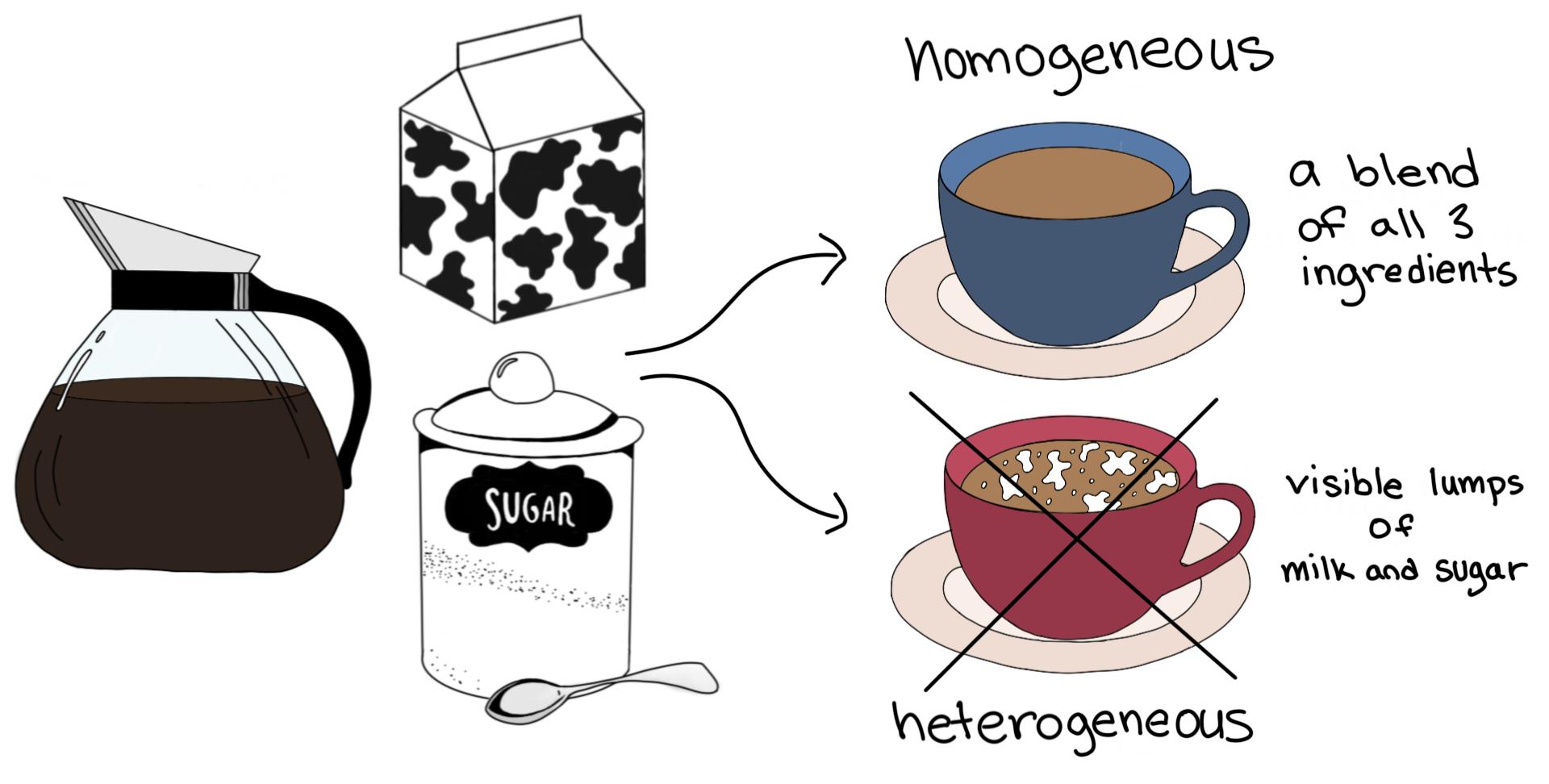



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Difference Between Homogenous And Heterogeneous Mixtures Brainly In




Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture
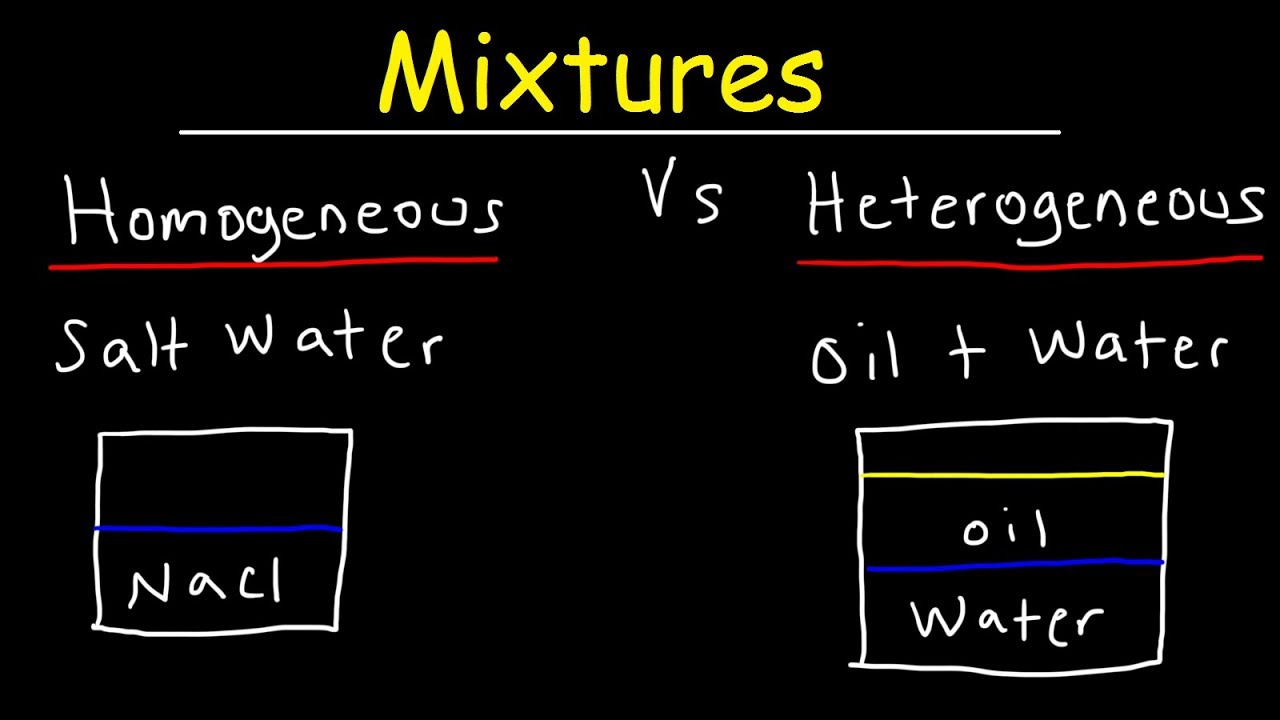



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube




Homogeneous Mixture




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




List The Points Of Difference Between Homogeneous And Heterogeneous Mixtures Brainly In




Heterogeneous And Homogeneous Mixtures Examples Pdf Homogeneity And Heterogeneity Mixture




1 Differentiate Between Homogon Eous Aid Heterogeneous Mixt Scholr
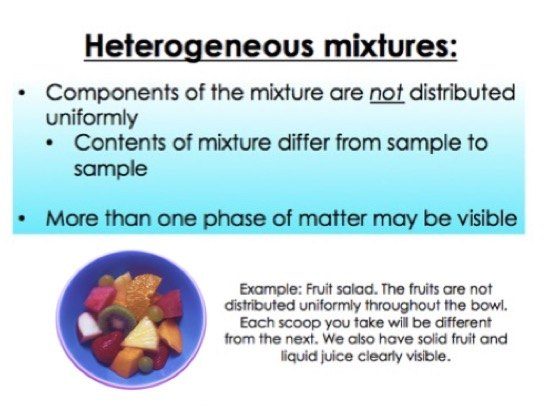



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Vs Heterogeneous Mixtures Difference And Comparison Diffen




Elements Compounds Mixtures Objectives 1 Explain The Difference Between An Element And A Compound 2 Compare Heterogeneous And Homogeneous Mixtures Ppt Download




Solutions And Mixtures Flashcards Quizlet




Difference Between Heterozygous And Homozygous Scholr



Elements Compounds And Mixtures Course Hero
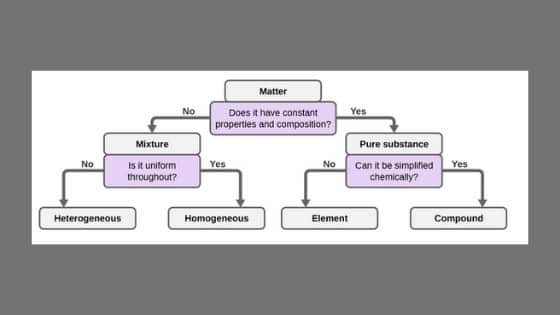



Homogeneous Mixture And Heterogeneous Mixture Ncert Books
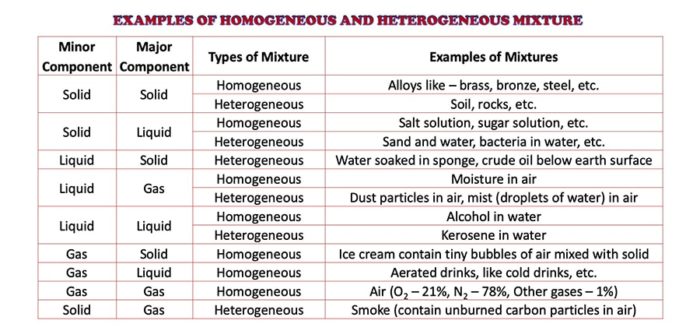



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Homogeneous Vs Heterogeneous Mixture Youtube



1




2 List Difference Between Homogeneous And Heterogeneous Mixture Youtube




What Is The Difference Between A Homogeneous And Chegg Com




What Is The Difference Between Heterogeneous Mixture Vs Homogenous Mixture Brainly Com




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




Classify Mixtures As Homogeneous Or Heterogeneous Worksheet



1
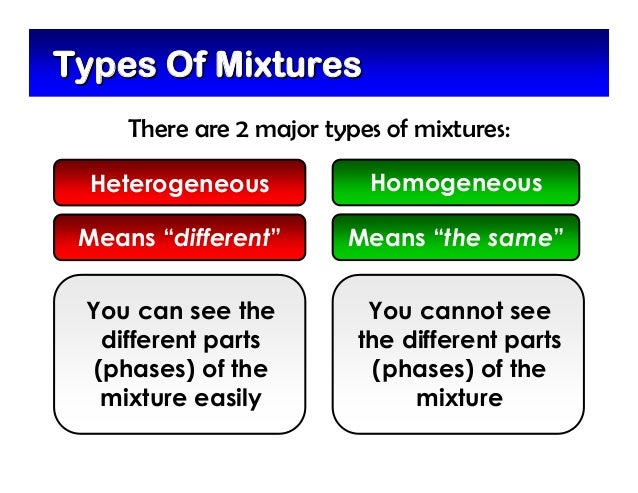



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com



Http Punainternationalschool Com Assets Upload Ck Images Class ix chemisty july aug material Pdf
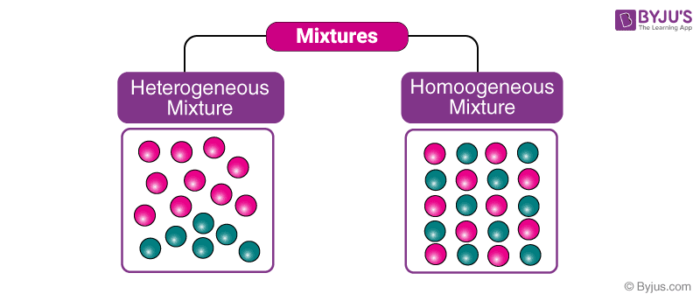



Heterogeneous And Homogeneous Mixture Differences Videos Examples




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms
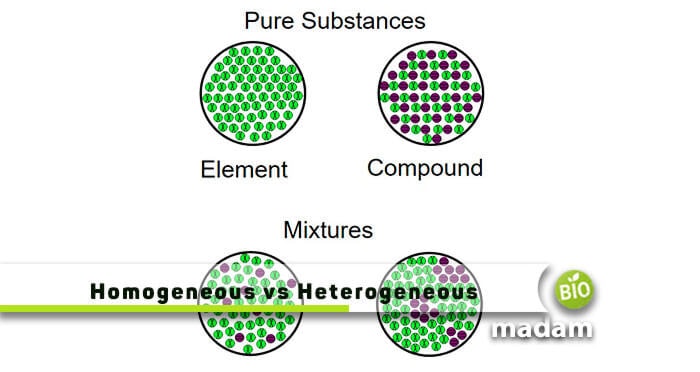



Difference Between Homogeneous Heterogeneous Mixtures Biomadam




List The Points Of Differences Between Homogeneous And Heterogeneous Mixtures



Sxxv4y Ycx9hem




Difference Between Homogeneous Mixture And Heterogeneous Mixture



Q Tbn And9gctxtzdpl9qyaaesytmccdhjgig7kctubdjyeec6sj2h Ew87kwi Usqp Cau




Difference Between Mixtures And Compounds In Tabular Form




Homogeneous Mixtures Vs Heterogeneous Mixtures By Valerie Calvo
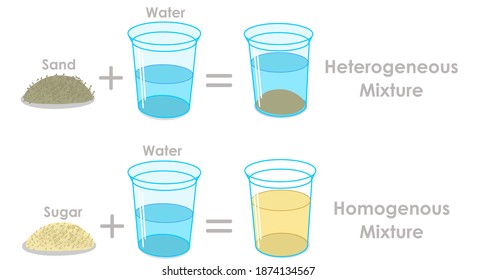



Mixtures Images Stock Photos Vectors Shutterstock
:max_bytes(150000):strip_icc()/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture Bitwise Academy




Heterogeneous Mixture And Homogeneous Mixture Youtube




G3wn 1qklkzplm




Compare And Contrast Homogeneous And Heterogeneous Mixtures Using The Venn Diagram Below In Filling Brainly Ph



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples



0 件のコメント:
コメントを投稿